
Under these conditions the rate of increase of the current with the distance between the plates was a maximum. The values of the ratio X/p were, 50 in pure helium, 200 in pure argon and 15 in the mixture. or acids to allow conduction of the electric current: Chemical equation. The three curves give the currents in pure helium, in pure argon and in helium containing 0.025 per cent of argon. Below 2.18 K, thermal conductivity of helium-4 becomes more than 1,000 times. 1, and the potential differences between the plates by the abscissæ. The photo-electric currents obtained with a constant force between parallel plates at different distances apart are represented by the ordinates of the curves, Fig. Abstract: The carry features of a quasi-one-dimensional surface electrons (Q1D-SEs) over the superfluid helium at the charged profiled substrate is. DefinitionThe electrical conductivity is defined as the quantity of electricity flowingper unit area per unit time at a constant potential gradient. In gases, the electrical conductivity in uniform fields between parallel plates depends upon the ratio X/p, where X is the electrical intensity in volts per centimetre, and p is the pres sure in millimetres of mercury, and is a maximum for a certain value of X/p depending upon the nature of the gas. The values of thermal conductivity demonstrating the impact of damage from pores and helium-filled pores are reported.
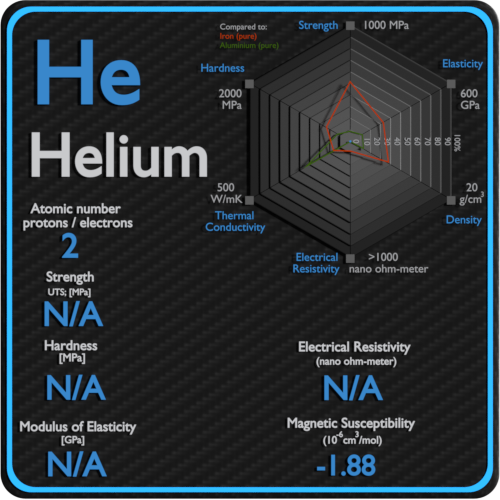
For example, the electrical conductivity of pure helium is greatly improved by the addition of 0.01 per cent of pure argon.

Superfluid helium has remarkable properties, including very high thermal conductivity it is an efficient heat conductor. However, if cooled below 2.17 K (-271.0C), it passes from the fluid to the superfluid state. Helium, superfluid or not, is a very good insulator. At atmospheric pressure gaseous helium becomes liquid at around 4.2 K (-269.0C). IT is well known that the electrical conductivity of certain gases may be greatly increased by the addition of very small quantities of other gases. 906 I fear this connection between the thermal and electrical conductive properties (the Wiedemann Franz law) only holds for metals where thermal conductivity is dominated by the contribution of the electrons.


 0 kommentar(er)
0 kommentar(er)
